Ariel Sanchez
A) Osteoporosis is defined as a skeletal disorder characterized by lower bone strength, which predisposes to fractures. Bone strength reflects the integration of 2 main characteristics: bone density and quality. Bone density is expressed in grams of mineral per area or per volume, and in any individual it is determined by the peak of bone mass achieved and by the amount of bone lost afterwards. Quality refers to architecture, replacement, accumulation of damage (eg microfractures), and mineralization. A fracture occurs when a force (trauma) is applied to an osteoporotic bone. This is why osteoporosis is an important risk factor for fractures, and a distinction must be made between risk factors for osteoporosis and risk factors for fractures.
B) It is important to correct the common misconception that osteoporosis always comes from bone loss. The loss of bone tissue usually occurs as men and women age; however, a subject who does not achieve optimal bone mass during childhood and adolescence can develop osteoporosis without accelerated bone loss. Hence, a suboptimal bone development during growth is as important as bone loss in the genesis of osteoporosis.
C) At the moment there is no exact measure of bone strength in general. Bone mineral density (BMD) is frequently used as a surrogate measure, since it explains approximately 70% of bone strength. The World Health Organization (WHO) gives an operational definition of osteoporosis as “a BMD 2.5 standard deviations (SD) below the mean of white women, young adults” (20-30 years). It is not clear how this diagnostic criterion applies to men, boys, and other ethnic groups. Due to the difficulty to achieve exact measurements and a standardization between different instruments (called densitometers) and different anatomical measurement sites (spine, hip, distal forearm, phalanges or metacarpals, tibia, calcaneus, etc.), there are controversies between experts with regarding the validity of this diagnostic criterion.
D) Osteoporosis can be classified as primary or secondary. Primary can occur in both sexes at any age, but it often occurs after menopause in women, and later in life in men as well. Secondary is the result of medications or other diseases (eg, glucocorticoid-induced osteoporosis, hypogonadism, or celiac disease).
E) Characteristic osteoporotic fractures are those that follow relatively low energy impacts (bending over, lifting a heavy object, falls from a low height, etc.). The incidence of fractures is high in people with osteoporosis and increases with age. The probability that a 50-year-old Caucasian person will have a hip fracture for the rest of their life is 14% for a female and 5-6% for a male. There is abundant studies showing that subjects who suffer fractures of any type, whatever their age and sex, have less BMD than healthy controls. So a history of fracture should arouse the suspicion of osteoporosis.
Pathophysiology
The progressive loss of bone mass after 40 years of age is an almost physiological phenomenon, which occurs in both sexes, in all latitudes and in all races, although it is less frequent in blacks. There is 30% less bone mass in the elderly (70-80 years) than in young people (20-30 years), the calcium content of the skeleton being lower in the former.
The process may preferentially affect trabecular bone or compact bone, which becomes more "porous". In osteoporosisc bone, the relationship between mineralized matrix and non-mineralized matrix is normal. In other words, although the volume of bone decreases, the bone that remains is adequately calcified.
There are three well-defined cell populations in bone tissue: osteoblasts, engaged in the apposition of new bone; osteoclasts, specialized in bone resorption; and osteocytes, mature cells that have lost their ability to synthesize bone and are capable, under special circumstances, of resorbing it. Bone formation and resorption are ongoing processes throughout life, in what is known as remodeling. This occurs on all surfaces of the bone: endosteal, periosteal, and intracortical or haversian. In general there is a close relationship between formation and resorption. The presence and type of activity on bone surfaces can be demonstrated by various techniques, including quantitative evaluation of cell classes in non-demineralized thin sections,
The balance between bone gain and loss is likely due to the coordinated activity of interacting "bundles" of cells in a relatively small volume of bone; This is what Frost called "basic multicellular units."
Most of the bony surfaces are generally inactive. Morphometric data obtained from iliac crest biopsies in normal adults indicate that the neoformation surface (covered with osteoid, that is, new bone not yet calcified) is 10% of the total surface, while the spring surface represents only a 2-4% of the total. Kinetic studies with Ca47 indicate that the pool of calcium (calcium in extracellular fluid and soft tissues, and interchangeable calcium in bone) is approximately 5 g. In normal adults, 0.5 g of calcium enters and leaves the bone each day. It can be calculated that 1/6 of the skeleton is resorbed annually, to be replaced again.
Mineralization is the process by which the inorganic mineral is deposited in the organic matrix. As the mineral is composed of calcium and phosphorus, the concentration of these ions in plasma and extracellular fluid influences its formation. Osteoblasts and osteocytes may be able to regulate the local concentration of calcium and phosphorus, and other important factors such as magnesium and pH. Collagen can catalyze the nucleation of the mineral phase from solutions containing Ca and P. However, it is believed that there are inhibitors capable of regulating mineralization in situ. Inorganic pyrophosphate is a potent calcification inhibitor. Alkaline phosphatase, present in osteoblasts, can catalyze the hydrolysis of pyrophosphate, and perhaps this enzyme has a role in regulating the deposit of mineral salts. The newly deposited mineral is amorphous, and its Ca / P molar ratio is relatively low. As it matures, it turns into crystalline hydroxyapatite. When calcium and phosphorus concentrations are high in the extracellular fluid, mineral can be deposited in tissues that usually do not calcify; on the contrary, if its concentrations are low, the bone mineral cannot form well.
The resorption process has not been sufficiently clarified, especially regarding the removal of ions from the matrix. This is dissolved by collagenases, but such enzymes cannot degrade the protein before it is devoid of the mineral phase.
Several hormones have profound effects on bone growth and remodeling. Parathyroid hormone (PTH) has almost immediate influences on bone cells, such as calcium influx into bone cells, activation of adenyl cyclase with intracellular production of cyclic adenosine monophosphate, and uptake of uridine and several amino acids. Osteoclasts are the preferred target for the action of this hormone, but its chronic effects involve, in addition to stimulating pre-existing osteoclasts, the formation of new ones. PTH also acts directly on osteoblasts, which have specific receptors. Much of the effect of PTH on osteoclasts is mediated by osteoblasts, which secrete, under the influence of PTH, cytokines with an effect on those cells.
The active metabolites of vitamin D, notably 1,25-dihydroxycholecalciferol or calcitriol, are also capable of increasing the activity and number of osteoclasts; its spring action is synergistic with that of parathyroid hormone.
The hypocalcemic action of calcitonin was known shortly after its discovery, and it seems to be due to direct stopping effects on osteoclasts. Inhibition of bone resorption is rapid onset, but disappears as soon as the hormone is removed. After the administration of calcitonin, increased levels of parathyroid hormone have been shown, probably stimulated by the decrease in plasma calcium.
Both osteoblasts and osteoclasts require the presence of thyroxine for their normal function. Hyperthyroxinemia increases both formation and resorption, and preferably the latter.
Glucocorticoids inhibit the transformation of precursor cells into osteoclasts in vitro, thus decreasing the resorption response of bone to parathyroid hormone. In vivo, excess of these steroids stimulates bone resorption, although this may be an indirect effect: as they decrease intestinal calcium absorption on the one hand and increase calciuria on the other, they induce a negative calcium balance that can lead to secondary hyperparathyroidism . On the other hand, glucocorticoids inhibit the expression of many growth factors with anabolic effect on osteoblasts; therefore, in metacorticoid osteoporosis, osteoblastic function is greatly diminished; corticosteroids delay the closure of fractures.
| Table 78-1. Humoral control factors of osteoblasts and osteoclasts | ||
|
Osteoblastos |
Osteoclastos |
|
|
Stimulate |
Mechanical tension, calcitriol, sex steroids, somatotropin, insulin-like growth factor 1, PTH |
Thyroxine, PTH, calcitriol, prostaglandins, tumor necrosis factor-alpha, other cytokines |
|
Inhibit |
Immobilization, old age, glucocorticoids |
Calcitonin, estrogens, bisphosphonates |
A signal system between bone cells has been known for a relatively short time: there is a factor in the membrane of cells of the osteoblastic lineage that is induced by factors stimulating resorption (eg, PTH). This ligand, a member of the tumor necrosis factor (TNF) superfamily, is called RANKL (nuclear factor kappa activator receptor ligand). It binds to a highly specific receptor, RANK, a transmembrane protein expressed by osteoclasts. The binding of this receptor with its ligand induces a cascade of intracellular events that lead to the differentiation of precursor cells into osteoclasts and the activation of mature osteoclasts. In addition, osteoblasts secrete a 401 amino acid propeptide that, when dimerized, becomes activated and can act as a "decoy" receptor; it is known as osteoprotegerin (OPG). It is a soluble receptor, not bound to the cell membrane, and that interposes between RANK and RANKL, preventing interaction between them and, therefore, inhibiting osteoclast activation. In other words, to differentiate and mature, cells of osteoclastic lineage must have direct contact with osteoblasts. The more RANKL and less OPG there are in a given microenvironment, the greater the number and activity of osteoclasts; Conversely, the less RANKL and more OPG are present, the fewer active osteoclasts (see Fig. 79-1). to differentiate and mature, cells of osteoclastic lineage must have direct contact with osteoblasts. The more RANKL and less OPG there are in a given microenvironment, the greater the number and activity of osteoclasts; Conversely, the less RANKL and more OPG are present, the fewer active osteoclasts (see Fig. 79-1). to differentiate and mature, cells of osteoclastic lineage must have direct contact with osteoblasts. The more RANKL and less OPG there are in a given microenvironment, the greater the number and activity of osteoclasts; Conversely, the less RANKL and more OPG are present, the fewer active osteoclasts (see Fig. 79-1).
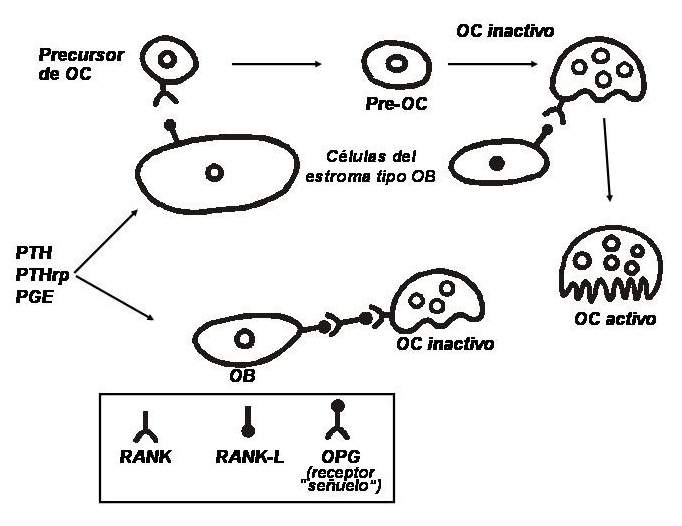
Regulation system between osteoblasts and osteoclasts. (Abbreviations: OB, osteoblast; OC, osteoclast; OPG, osteoprotegerin; RANK, kappa nuclear factor activator receptor; RANKL, RANK ligand; PTH, parathyroid hormone; PTHrp, PTH-related peptide; PGE, prostaglandin E).
Sex hormones have a physiological role in controlling resorption. In vivo, the administration of estrogens or androgens reduces bone resorption and favors a positive calcium balance, at least in the short term. Estrogens have been postulated to make osteoclasts more refractory to the action of parathyroid hormone. There are estrogen receptors on bone cells of the osteoblast line.
Considering the main factors that regulate the dynamics of remodeling, one fact seems incontestable: for osteoporosis to occur, bone resorption must exceed formation. This can happen in any of the following situations:
a) formation decreases while resorption continues its usual rate;
b) formation continues normally, but resorption is accelerated.
Different techniques have provided arguments for the two hypotheses, but it is most likely that an annual loss of skeletal mass greater than 1%, capable of producing clinical manifestations, is due to a combination of mechanisms:
e) bone formation decreases and resorption increases.
Table 78-2 gives a classification of the different osteoporosis according to its cause. If a disease process is identified to which osteopenia can be attributed (eg, hyperparathyroidism, or hypercortisolism), it is considered “secondary”. If not, it is concluded that it is a “primary” or “idiopathic” osteoporosis, of which a very rare juvenile type is recognized, which mainly affects men between 20 and 40 years of age, and the most common form, the Involution or senile osteoporosis, which predominantly affects women after 50 years of age, also receives the adjective "postmenopausal".
Table 78-2
The pathophysiology of the latter is the best studied and is summarized in Figure 79-2.
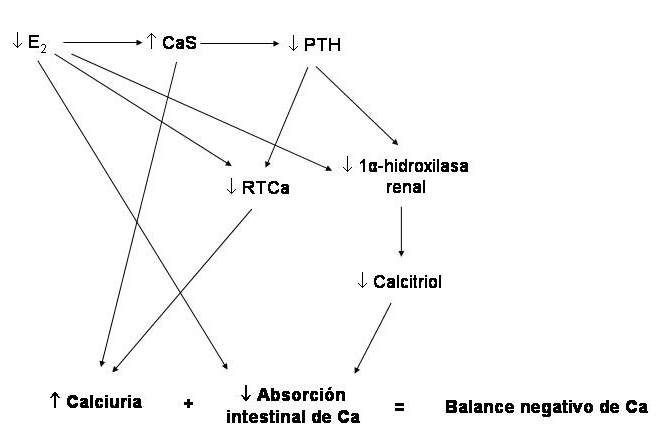
Figure 78-2: Pathophysiology of postmenopausal osteoporosis. (Abbreviations: E2, estradiol; PTH, parathyroid hormone; RTCa, tubular calcium reabsorption).
By decreasing the estrogen content due to the cessation of ovarian function (castration, menopause), there is derepression of the synthesis of numerous cytokines of lympho and monocytic origin (the main ones are interleukins 1 and 6, and tumor necrosis factor alpha), that activate the osteoclasts and, therefore, cause greater spring activity, with a slight increase in calcium levels, which slows down PTH secretion. The consequences are an increase in the tubular reabsorption of phosphorus and a decrease in that of calcium. There is an increase in serum phosphorus and urinary calcium; hypercalciuria is accentuated by lack of stimulation of tubular calcium reabsorption normally exerted by estrogens. Both the decrease in circulating PTH and the increase in phosphataemia inhibit the activity of renal 1-alpha-hydroxylase, with lower production of calcitriol and, consequently, lower intestinal absorption of calcium. In addition, it decreases due to lack of direct stimulation of estrogens. The calcium balance tends to be negative. With small negative balances, nothing happens in the short term, but if the situation continues for months and years, the deposit from which the calcium has been extracted - the bone - will suffer the impact of the cumulative loss (example: -20 mg / day for 3 years = -2.2 kg of calcium). Another important aspect of the pathophysiological spectrum of this disease is apoptosis (programmed cell death) suffered by osteoblasts and osteocytes due to the lack of estrogens and the increase in the cytokines mentioned above. With this, bone formation decreases, and the microdamage repair process in bone tissue is less efficient.
The pathophysiology of senile osteoporosis is somewhat different: in the elderly, the lower generation of vitamin D in the skin due to the effect of the ultraviolet rays of sunlight, the decreased synthesis of calcitriol by the kidney, and the lower efficacy of intestinal absorption of calcium, with less ability to adapt to calcium-restricted diets. This leads to secondary hyperparathyroidism, which accelerates bone turnover. The relative deficit of growth hormone in the elderly, and the lower systemic and local generation of insulin-like growth factor 1 (IGF-1) probably also play a role.
|
Table 78-3. Causes of osteoporosis
|
Symptoms and signs
In osteopenic syndrome the symptoms are generally vague and unspecific, and there are no "pathognomonic" signs.
Generally, the patient complains of diffuse, poorly defined bone or joint pain, which is most often located in the spine. When a vertebral fracture (crush) occurs - with or without triggering stress or trauma - the pain is intense and localized, and may radiate to the anterior part of the chest or abdomen, depending on the height of the affected vertebra. There may be mild or moderate chronic low back pain.
In subjects who have suffered the collapse of more than one vertebra, there is a decrease in height, which is less than the wingspan (distance that separates the ends of the larger fingers of both hands, with the arms extended in a cross). There is a decrease in the vertex-pubis distance with respect to the pubis-sole, which are normally equal. The lower point of the costal ridge approaches the iliac wing and may touch it; in addition, the arms at rest appear longer, with the hands closer to the knees (Fig. 78-3).
When two or more consecutive dorsal vertebrae are crushed, the angulation of the spine leads to an exaggeration of the physiological dorsal kyphosis, with the appearance of a hump. In addition, there is a deformity of the entire rib cage, and a compensatory exaggeration of the cervical lordosis.
Although vertebral fractures, like those of the femoral neck, are seen more frequently in elderly patients, in women and during the first years after menopause it is more common to see wrist fractures (distal third of the radius and ulna).
Except in the case of recent fractures, there is no pain from the pressure of the bones by the observer. Otherwise, patients with primary osteoporosis are in good general condition. In contrast, those with secondary osteoporosis often have the symptoms and signs of associated diseases.
F) In premenopausal women with osteoporosis, it is believed that 1 in 2 has a secondary form of this disease, the most common causes being hypoestrogenism, corticosteroids, excess thyroid hormone (endogenous or exogenous) and anticonvulsant therapy. In postmenopausal women, the prevalence of secondary causes is surely much lower, but the actual proportion is not known. Hypercalciuria, hyperparathyroidism, and malabsorption are causes to be taken into account and easy to confirm or rule out (requesting calcemia, 24-hour calciuria, stool digestibility study, antigliadin and antiendomysium antibodies, for example). In other words, complementary studies in postmenopausal women with osteoporosis may be necessary in individual cases,
G)
Study methodology
H) A good medical history and a complete physical examination are essential in the evaluation of the risk of fracture, and height measurement should be included: height losses> 3 cm are an indication of vertebral crushing, as is dorsal kyphosis.
Radiology allows an appreciation, albeit crude, of the magnitude of the process, which causes an increase in the transparency of the bones. To quantify it more accurately, different methods are used. One, evaluating cancellous bone, requires radiographs of both femoral heads to compare the degree of “untrabeculation” with a standard (Singh's index; Fig. 78-4). Another, useful for evaluating compact bone, establishes the cortico-medullary relationship in long bones; The second metacarpal of the non-dominant hand is generally used (Garn radiogrammetry). Normally, the sum of the thickness of both cortices should be greater than or equal to the diameter of the medullary canal; as compact bone is lost, the diaphyseal cortex thins and the diameter of the medullary canal increases.
The dorsal vertebrae, when yielding their structure in the face of compressive forces, adopt - when seen in profile - a wedge shape; the lumbar, due to generalized protrusion of the intervertebral discs, take on a biconcave shape, like fish vertebrae (Figs. 72-5 and 72-6).
Other methods that are much more exact, precise and non-invasive allow the estimation of bone mineral content: radiological densitometry and quantitative computed axial tomography measure the attenuation of a single or double beam of photons passing through the body. This allows determination of calcium content in the lumbar spine, proximal femur, or other regions (Fig. 78-7).
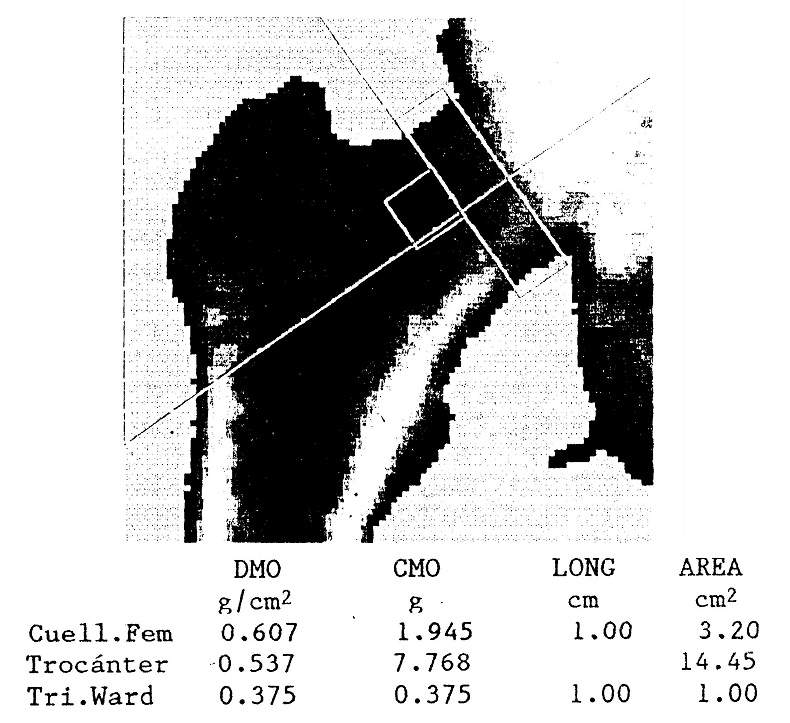
Figure 78-7: Densitometric image of the proximal extremity of a right femur, studied by dual X-ray absorptiometry. Bone mineral content (CMO, in grams), area (in cm2) and bone mineral density (BMD) are reported , in g / cm2) of the femoral neck, greater trochanter, and Ward's triangle.
Ultrasound is also used to assess the quality of the peripheral bone (the site generally studied is the calcaneus). Densitometry strongly correlates with resistance to fracture of the hip and vertebrae in biomechanical tests, and it also accurately predicts the risk of fracture. Different skeletal sites and total skeletal mineral content can be evaluated, although whole-body densitometry is reserved for special cases and clinical investigations.
I) For the diagnosis, the criteria of a WHO expert committee is used, which uses the T value (defined as the number of SDs above or below the mean corresponding to healthy young women). The T value must be distinguished from the Z, which is defined as the number of SDs above or below the mean corresponding to subjects of the same age and the same sex. According to the WHO definition, osteoporosis is present when the T value is less than -2.5 SD, or even more negative. If the value is positive or greater than -1, the bone mass is considered normal, and if it is between -1 and -2.49 it is said that there is “osteopenia”. If the value corresponds to osteopenia, but fragility fractures already exist, the diagnosis of osteoporosis is made. Although this criterion was originally based on measurements made at the hip with dual x-ray absorptiometry (DEXA), it has been extended to evaluate other skeletal sites, and using other technologies. There are doubts among experts as to whether this generalization provides the same diagnostic and predictive information. Measurement at the hip is better at predicting risk of hip fracture than measurement at other sites, just as measurement at the lumbar spine is better at predicting risk of vertebral crush.
J) From the point of view of diagnosis and the evaluation of many subjects, the use of simpler devices, sometimes portable, that provide quick information and at a lower cost, such as DEXA of the distal forearm or calcaneus, or ultrasound of the calcaneus, is acceptable. phalanges or calcaneus. However, experts agree that a “osteoporosis” diagnosed at a peripheral site must be confirmed by a measurement at an axial site, by DEXA or quantitative computed tomography. Furthermore, peripheral densitometry is not accepted as valid for monitoring bone response to treatment with osteoactive drugs.
K) The laboratory can also provide data indicating the turnover with the so-called “markers”: total alkaline phosphatase or its serum insoenzyme of bone origin and osteocalcin give an idea of the rate of new bone formation (osteoblastic activity), while the Urinary pyridinoline and deoxypyridinoline, and type I collagen peptides (CTX, NTX) measured in serum or urine, give an idea of the rate of bone resorption (osteoclastic activity). These markers do not make the diagnosis of osteoporosis, but indicate whether turnover is high or normal and can better target the type of underlying disorder; therefore, they help to choose the treatment. In addition, as they vary according to the variation of the part, They allow evaluating the response to treatment in shorter periods (weeks or months) than those necessary to measure changes in bone mass (minimum 1 year). In elderly subjects, and especially in institutionalized ones who do not live outdoors or expose themselves to the sun, the determination of calcidiol (25-hydroxyvitamin D) in serum and, if the value is low, that of serum PTH is extremely useful. , since secondary hyperparathyroidism is a common cause of senile osteoporosis and its prevalence is high in the elderly.
In short, when faced with a patient with osteoporotic syndrome, the doctor's efforts must be directed towards ruling out the correctable causes (malnutrition, hyperthyroidism, hyperparathyroidism, etc.).
In every patient over 50 years of age who suffers a fracture, the bone mass must be evaluated with objective methods, and then preventive or curative measures are taken. Bone density should also be measured in people with two or more of the risk factors listed in Table 2.
|
Table 2. Risk factors for osteoporosis
|